Write the Rate Law for the Iodine-clock Reaction.
X Read the lab thoroughly. Write the rate law for this version of the iodine clock reaction.
Write a rate law for the iodine clock reaction based on your experimental data.
. Between the Reaction Rate and Reagent Concentration takes a simple form known as the Rate Law. The rate or speed of the reaction is dependent on the concentrations of iodide ion I- and hydrogen peroxide H 2 O 2. Of Physical Sciences Engineering Wilbur Wright College concentrations change.
Could the rate law have been predicted using the coefficients in the balanced chemical equation. The spectator ions are left off the reaction Therefore we can write the Rate Law concentration dependence for the reaction as. Klautzsch 5 1 1 for Trial K T 295 K 7 Temperature in Kelvin K.
The rate law now is. If iosate concentration was double what would happen to the overall rate of the. Derive units for the rate constant kbased on the rate law you determined in this experiment.
The rate law for this reaction will be in the form. Iodine Clock Reaction Chemistry 203 General Chemistry II Dept. Rate k A n B m Eq.
The reaction rate doubles. How do I set up the rate law for this reaction. The values for m and n are typically whole numbers that is used to find the overall order of the reaction mn.
The slope represents the order of reaction with regards to the iodate but the actual value is 20. What is the overall rate order of the reaction. In this experiment we will examine the effects of both temperature cold and hot and the effect of a catalyst on the rate of the reaction.
Rate k I x H 2 O 2y 2. Up to 24 cash back concentration of A stays constant and Reaction Rate doubles. Ratei kH2O2i xH i yI- i z In this experiment we will find x by varying the concentration of H2O2i while holding H i and.
The rate or speed of the reaction is dependent on the concentrations of iodide ion I- and hydrogen peroxide H 2 O 2. We can write a generic. RatekI- 2 S 2 O 8 2- Yes because the coefficients match the order of the reaction.
In order to relate the rate of a reaction to the factors discussed above chemists write rate laws for reactions. It follows that for this reaction the Rate Law is. When it was doubled the reaction was significantly slower for obvious reasons.
It follows that the initial rate is directly proportional to the initial concentration of B. Equation of the line of best fit is y 2068x 1213. The rate law of a chemical reaction is a mathematical.
The spectator ions are left off the reaction Therefore we can write the Rate Law concentration dependence for the reaction as. Rate k I x H 2 O 2 y 2. Rate k ceH_2O_2ceI- label2 That is the reaction is first order with respect to both hydrogen peroxide and iodide.
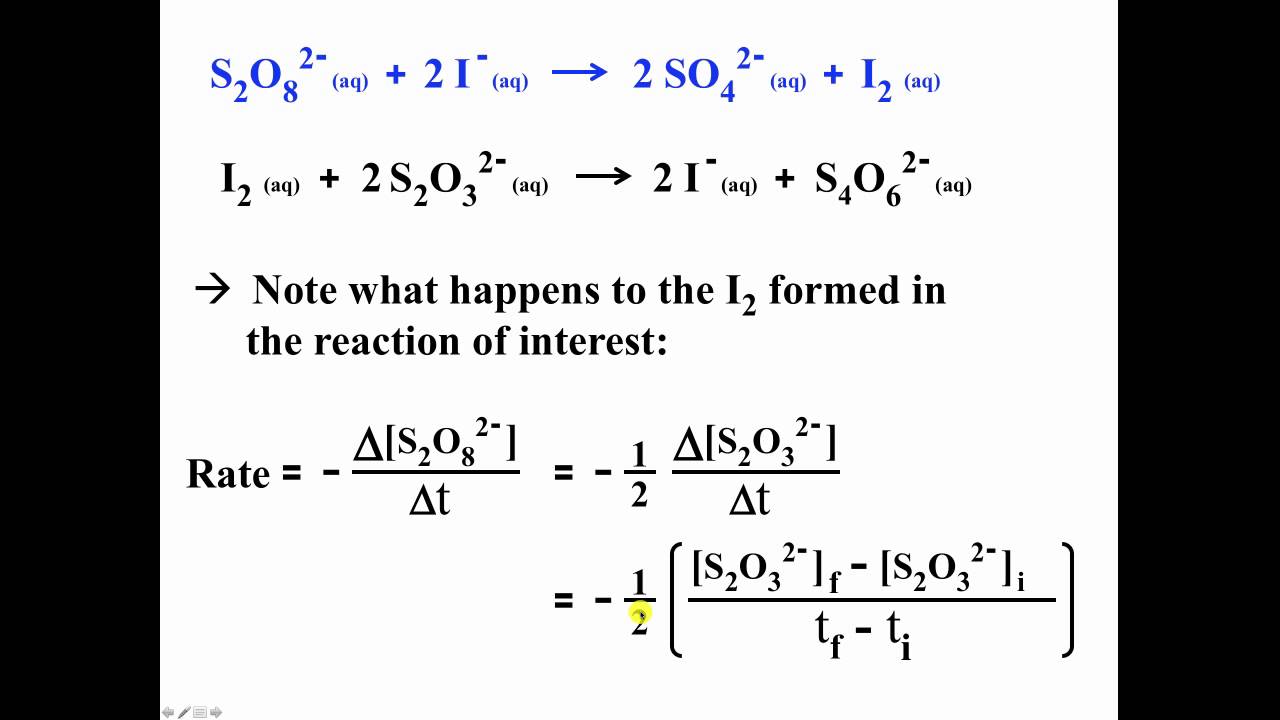
The iodine clock reaction part michael burand oregon state. When does the color change occur in the Iodine Clock Reaction The color change occurs as soon as all S₂O₃² reacts and a buildup of I₃ causes a color change. Therefore if our data tells us that the value for m is 2 and the value for n is 1 then we know that the rate of the overall reaction is 213.
3 where A and B are generic reacting Species k is a reaction specific proportionality constant known as the Rate Constant and n and m are the Reaction Order. However the reaction is not just dependent on those chemicals its also dependent on the ceS2O32-. 8 2- n 3.
The spectator ions are left off the reaction Therefore we can write the Rate Law concentration dependence for the reaction as. Which relates the rate of the reaction in units of Msec to the concentration of reactants. Rate k Ix H 2 O 2y 2.
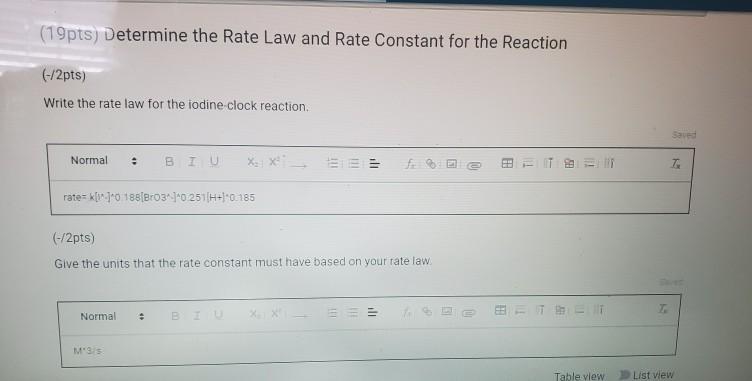
Usually you take the slow step and set up an equation based on that like textrateceH2O2x I- y. CH 262 W14 Rate Laws Part 1 Burand V1 oregon state university ch 262 winter 2014 burand rate laws. 19pts Determine the Rate Law and Rate Constant for the Reaction 2pts Write the rate law for the iodine clock reaction Normal ΒΙΙΙΟ EEE 2pts Give the units that the rate constant must have based on your rate law.
The rate or speed of the reaction is dependent on the concentrations of iodide ion I- and hydrogen peroxide H 2 O 2. The x- and y-values are what you will be determining in this experiment. Previous determinations have found that the rate law for this reaction is.
Rate k IO 3 x HSO 3 y Equation 5 where k is the rate constant for this reaction at a particular temperature and x and y are the reaction orders for the iodate and bisulfite ions respectively. Iodine Clock 2 The rate law The general rate expression for this reaction is Rate Ms kH2O2 xHyI-z The initial rate of the reaction can be expressed in terms of the initial concentrations of the reactants. The reaction is 1st order with respect to persulfate.
What was the rate law and overall reaction order for the iodine clock reaction. Rate Law k A1B1. The reaction is therefore of the first order with respect to B and n 1.
Normal BII U f Table view List View Report rate constant values to three significant figures. The Kinetics of the Iodine Clock Reaction 20 Experiment 2 The Kinetics of the Iodine Clock Reaction Pre-lab Assignment Before coming to lab. Rate k I- m S.
The Rate Law parameters k n and m must be determined experimentally. The order of the reaction with respect to each substance must be determined first refer to figures Rate Rate Constant for k M s 40 002 M 008 M 6 simply divide one the temperature in kelvin. Then write a ratio of rate laws placing the experiment with the larger rate in the numerator rate k S 2 O 8 2- m I - a.
For this experiment we will be studying a reaction known as the iodine clock. Thus the percent error for the order of reaction with regards to the iodate is 34.

How To Do Lab Report Exp 004 Rates Of Reaction For Iodine Clock Reaction Youtube

Solved 19pts Determine The Rate Law And Rate Constant For Chegg Com

Kinetics Of The Iodine Clock Reaction Intro Theory Youtube

Solved 2pts Write The Rate Law For The Iodine Clock Reaction Saved Normal 8 I U Xlxi 61616 Zt 8 Fxl 0 01 Rate Kd Broz H 2pts Give The Units That The Rate Constant Must
No comments for "Write the Rate Law for the Iodine-clock Reaction."
Post a Comment